Research
The molecular mechanisms of the systems linkage between signal transduction and RNA regulation will be elucidated, focusing on the analysis of animal and cellular models of heart failure, acute lung injury and malignant tumor diseases.
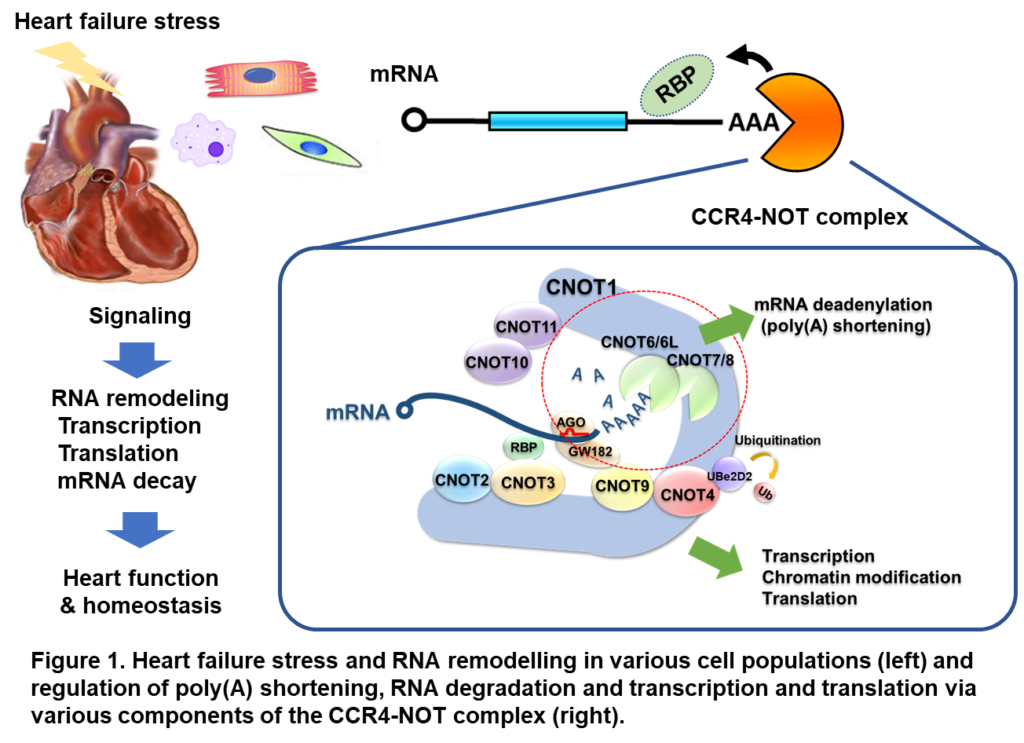
Mechanisms of RNA metabolic regulation in the control of cardiovascular function and therapeutic applications.
There is a theory that RNA is the origin of life. However, it had been thought that proteins were synthesized based on the genetic information in DNA in a DNA→RNA→protein flow, and that RNA was merely a mediator of genetic information. In recent years, it has become clear that RNA is a new regulatory hierarchy for gene expression. We are trying to understand the principles of life and disease that cannot be explained by transcription or epigenomics in terms of the regulatory mechanisms of RNA synthesis, degradation and translation: the CCR4-NOT complex acts as an executioner of the poly(A) strand of RNA, and the CCR4-NOT complex is a key regulator of the RNA synthesis, degradation and translation of RNA. Previously, we identified the CCR4-NOT complex as an essential factor for the maintenance of cardiac function in an RNAi screen (Cell 2010). We then elucidated that poly(A) degradation is important for the maintenance of cardiac function and involved in the transcriptional regulation of autophagy molecules (Science Signaling 2018). In the course of these studies, it has become clear that the CCR4-NOT complex is not only an RNA degrading factor, but also influences the regulation of transcription and translation, although the details are unclear. It has also been found to be involved in energy metabolism and nuclear homeostasis. Through the analysis of genetically engineered mice for each subunit within the CCR4-NOT complex, our laboratory aims to clarify how RNA degradation and transcription/translation affect each other in the regulation of cardiac gene expression and physiological functions.
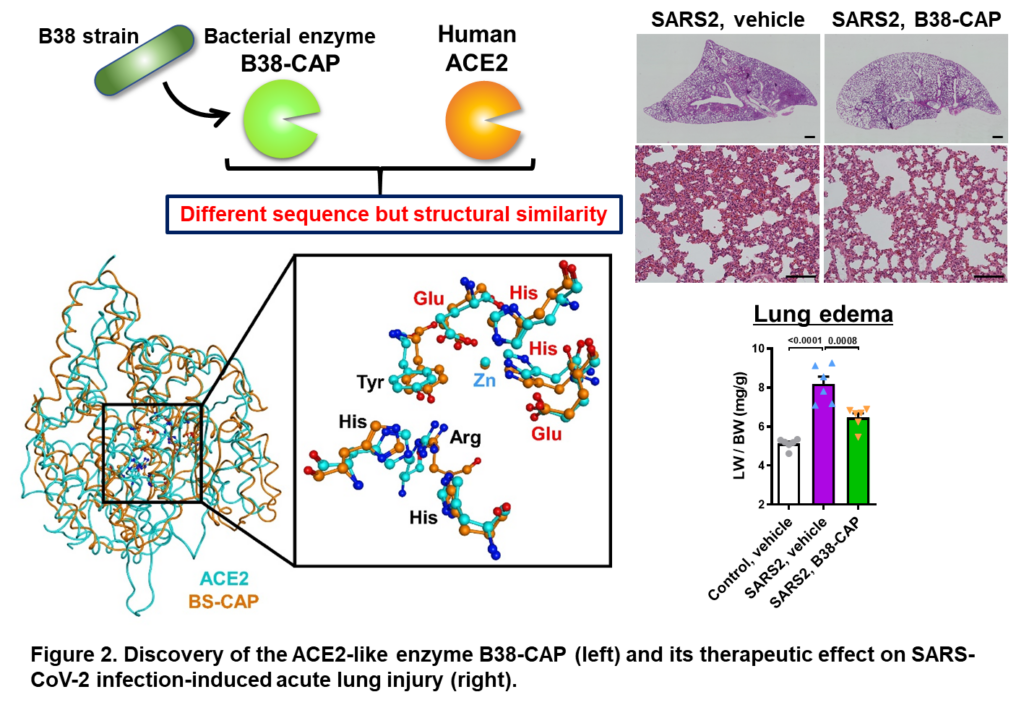
Pathogenesis of severe acute lung injury and sequelae development caused by COVID-19 or highly pathogenic influenza.
SARS-CoV-2 infection (COVID-19) leads to a wide range of symptoms, from asymptomatic, mild cold-like symptoms to severe cases requiring ICU care, and even patients with mild or moderate disease have severe subjective symptoms and are at risk of sudden death. Furthermore, post-corona sequelae, such as general fatigue and abnormalities in the sense of smell and taste, which persist after recovery, are also major problems. To solve these problems, other research approaches, such as detailed pathological analysis using animal models of infection, are essential to elucidate the molecular mechanisms underlying the onset of severe illness and sequelae. Angiotensin-converting enzyme 2 (ACE2) is a well-known receptor for the novel coronavirus (SARS-CoV-2). We demonstrated that ACE2 is a receptor for the 2003 SARS coronavirus and that its enzymatic activity to degrade angiotensin II inhibits the exacerbation of inflammation in acute respiratory distress syndrome (ARDS) (Nature 2005, Nat Med. 2005). Subsequently, we discovered the microbial ACE2-like enzyme B38-CAP (Nat Commun 2020), which demonstrated that the enzymatic activity of ACE2 is important in reducing the exacerbation of ARDS by SARS-CoV-2 (Nat Commun 2021). Currently, the rapid mutation of the virus is demonstrating the critical importance of understanding factors on the host side of infection. Our laboratory aims to elucidate the mechanisms of severe illness, sudden death and sequelae caused by neurovascular invasion of SARS-CoV-2 by analyzing the detailed molecular pathogenesis using a model of severe SARS-CoV-2 infection, and to establish a platform for the development of rapid therapeutic agents in the era with coronavirus.
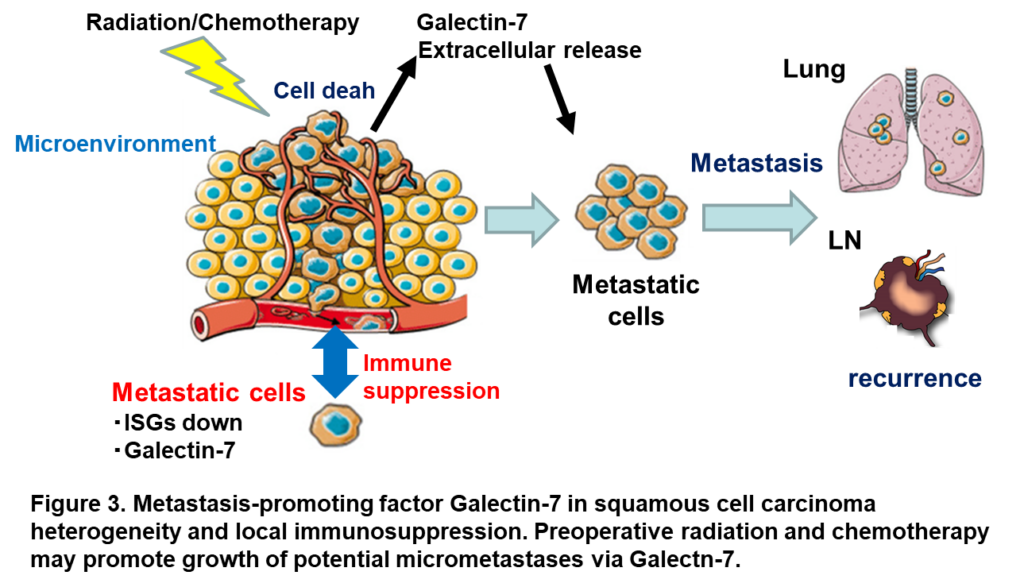
Molecular mechanisms of tumor immune heterogeneity and acquisition of cancer cell invasive and metastatic potential.
Esophageal squamous cell carcinoma is a malignant tumor with a poor prognosis, and despite preoperative radiotherapy and chemotherapy killing many of the cancer cells of the primary tumor and curative surgery, recurrence due to metastasis is a problem in some cases. It is known that the tissue of primary tumors shows complex heterogeneity as the disease progresses and that a specific cancer microenvironment is important for invasion and metastasis. Our laboratory has previously identified Galectin-7 as a metastasis-promoting factor by performing spatial transcriptome analysis in primary tumor tissue of a mouse squamous cell carcinoma cell metastasis model, and found that Galectin-7 does not affect the size of the primary tumor, but is a specific metastasis-promoting Galectin-7 may be released by cancer cells as damage-associated molecular patterns (DAMPs) in the in vivo cancer microenvironment and may be released by preoperative radiation and chemotherapy-induced cell death and damage of cancer cells (Oncogene 2022). Galectin-7 may be secreted and reverse the metastatic activation of the few surviving cancer cells. We are currently analyzing the molecular mechanisms of the regulation of tumor immunity by Galectin-7 and at the same time examining its potential for new predictive and therapeutic applications against cancer metastasis.